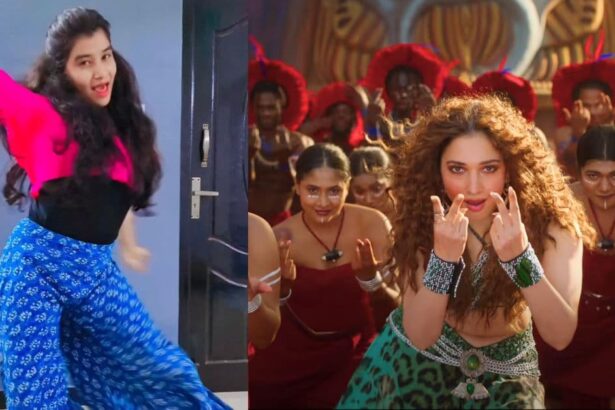Quick Links
Search
© 2023 Shoppersvila. All Rights Reserved.
Is Meri Brown Currently in a New Relationship? Know Who is Meri Dating Now?
Meri Brown was part of a polygamist family for over 30 years as the first…
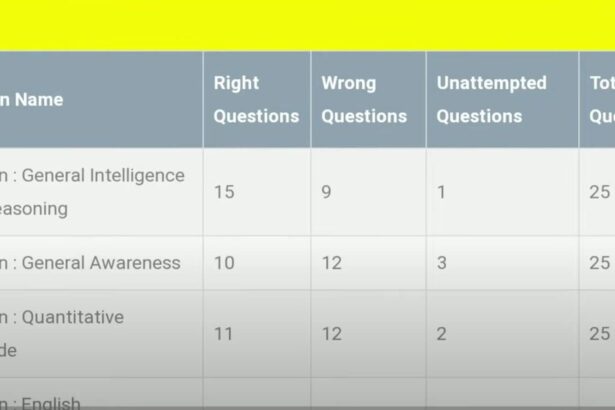
Rankiq SSC MTS 2023: How to Check SSC MTS Rank and Normalization in Rank Iq?
Do you checked answer key of SSC MTS 2023 exam recently and curious about how…
Don't Miss
Bigg Boss Star Dhanalakshmi Joins the ‘Kaavaala’ Fever with Her Energetic Dance Moves
The sizzling dance performance of actress Tamannaah to the song "Kaavaala" from Rajinikanth's Jailer has…
Hollywood
Explore This Category
Is Camryn Manheim Gay or Lesbian? The Truth About All The Rumors
For years, acclaimed actress Camryn Manheim has faced speculation about her sexual orientation. Fans often…
Truth Behind Molly Qerim and Stephen A Smith Relationship
Molly Qerim and Stephen A. Smith are two of the biggest names at ESPN, as…
Bollywood
Explore This Category
Adventure
Explore This Category
Top 10 Most Daring Motovloggers in Tamil Nadu
Tamil Nadu's motovlogging scene is thriving, with talented content creators taking to two wheels to…
What Happened to Popular YouTuber TTF Vasan? Is he Alive?
Popular Tamil YouTube star TTF Vasan has been critically injured in a horrific bike accident…
Who is TTF Vasan? Has TTF Vasan (Twin Throttlers) Died in a Bike Accident?
TTF Vasan, the wildly popular Tamil YouTube motovlogger renowned for his death-defying bike stunt videos…
Who is the No. 1 Motovlogger in Tamil Nadu?
Tamil Nadu has a thriving community of talented motovloggers. But one star stands out from…
Stay Connected
Discover Categories
Discover More of What Matters to You:
First AI vs Human Pilot Dogfight: Who Won the AIR Battle?
The US Air Force made history with the first-ever combat test between an AI-controlled aircraft and a human pilot. Conducted…
Here is Why Powerball Winning Numbers Trending in 2024
The year 2024 has seen an unprecedented level of public interest and social media engagement around Powerball winning numbers. With…
Created These Stunning Mother and Baby Unicorn AI Images with DALL-E 3
These breathtaking images were generated using the latest version of DALL-E 3.0, a cutting-edge AI image creation tool that has…
Anushka Sharma Returns Home: Son’s Privacy Prioritized
After a brief absence, Bollywood star Anushka Sharma has returned to India with her newborn son Akaay, making a notable…
A Diamond in the Rough: Finding Samurai in Aladdin?
Disney's animated classic "Aladdin" whisks us away to a vibrant tapestry of Arabian nights, filled with magic, adventure, and romance.…
21 Savage Accused of Scamming Adin Ross With Marked Cards
Rapper 21 Savage is facing backlash after being accused of cheating popular Twitch streamer Adin Ross with marked cards during…
Quaden Bayles Controversy on Bullying Scam: Know the Story
In February 2020, a heartbreaking video of 9-year-old Quaden Bayles went viral globally, bringing attention to the devastating impacts of…